Abstract
Daratumumab (DARA) has shown efficacy in patients (pts) with AL amyloidosis inducing rapid and deep hematologic and organ responses. DARA with VCd is an approved treatment for newly diagnosed pts with AL amyloidosis, while DARA-based therapies are a major salvage option for pts with relapsed or refractory AL amyloidosis. However, with the increasing use of DARA, there is a growing number of pts who either relapse or are refractory to DARA-based therapy. The data on the management of these pts are scarce and treatment options may be limited. In the current analysis, we aim to describe the outcomes of pts with AL amyloidosis who failed on DARA treatment and received further salvage therapy.
The analysis included consecutive pts with AL amyloidosis treated in the Department of Clinical Therapeutics, Athens Greece, who received DARA-based therapy, at any time during the course of their disease. Overall, 116 pts with AL amyloidosis received therapy with DARA and the analysis focused on 31 (26.7%) that either relapsed after DARA discontinuation or were refractory to DARA or had non-satisfactory response requiring further therapy.
The characteristic of these 31 pts at the time of initial diagnosis, included cardiac involvement in 27 (87%), renal in 15 (48%), liver in 4 (13%), PNS in 5 (16%) and 9 (29%) had soft tissue involvement. Baseline Mayo stage was 3% / 45% / 48% for stages 1 / 2 / 3 [6 (19%) were Mayo 3b] and renal stage distribution was 55% / 32% / 10% for stages 1 / 2 / 3. Translocation (11;14) (in 29%), and +1q21 (in 26%) of pts were the most common cytogenetic abnormalities.
At DARA index therapy, 23 (74.2%) pts had received DARA monotherapy and 8 (25.8%) in combinations (mainly with bortezomib); in 13 (42%) pts as first line and in 18 (58%) as salvage therapy; the median number of treatments prior to DARA index therapy was 1 (range 0-4). DARA was given as salvage therapy in 11 (61.1%) pts due to inadequate hematologic response to prior therapy, in 6 (33.3%) due to hematologic relapse or progression and in 1 (5.6%) due to organ progression. Median duration of DARA therapy was 2.8 months (range 0.5-31.7 months) and on ITT the hematologic response rate at index DARA therapy was 61.3% [CR/VGPR: 10 (32.3%), PR: 9 (29%)].
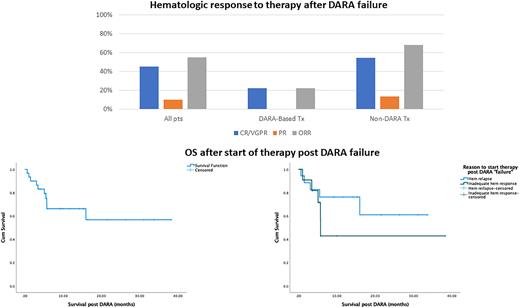
DARA failure was due to hematologic relapse in 18 (58%) pts, inadequate hematologic response in 11 (32%) and in 2 (6.5%) due to organ progression; the median time to "failure" during or after therapy with DARA was 13.6 months (0.5-43.6); at the time of DARA failure, 8 (26%) pts had had an organ response and 5 (16%) organ progression. At the time of DARA failure, 26 pts (84%) were exposed to bortezomib, 15 (48%) to lenalidomide and 3 (10%) to melphalan. Treatments post DARA failure included re-treatment with DARA combinations (with bortezomib or IMiDs) in 9 (29%) pts, venetoclax in 3 (9.7%) pts, belantamab mafodotin in 4 (12.9%) pts, bortezomib-based regimens in 6 (19.4%) pts, lenalidomide-based in 3 (9.7%) pts, pomalidomide in 3 (9.7%) pts, ixazomib in 2 patient (6.4%) and alkylating agent in 1 patient (3.2%). Median NTproBNP at start of post DARA therapy was 3936 ng/L and median dFLC was 123 mg/L. Hematologic response rate was 55% [VGPR/CR: 14 pts (45%), PR: 3 pts (10%)]; organ responses were observed in 9 pts (29%) and organ progression in 16 pts (51.6%). Among the 9 pts retreated with DARA-based regimens, 2 (22.2%) achieved VGPR and for non-DARA regimens hematologic response rate was 68.1% [CR/VGPR: 12 (54.5%), PR: 3 (13.6%)]. Ten pts (32.3%) have died; 1- and 2-year survival rate from the start of post DARA therapy was 64% and 55%. In univariate analysis, factors that were associated with inferior survival post DARA were NTproBNP (p=0.002), ALP (p<0.001) and dFLC (p=0.025) levels. Pts that failed DARA due to inadequate hematologic response had inferior survival than those that started therapy due to hematologic relapse, but the difference was not statistically significant (p=0.279) (Fig 1).
In conclusion, treatment of pts with AL amyloidosis who have failed DARA therapy is feasible, based on the use of non-cross resistant drugs or other targeted therapies. Hematologic responses in 55% and a 2-year survival of 55% in this difficult to treat population are respectful and set the benchmark for the evaluation of new therapies in this setting. Re-treatment with DARA may not be the best option, but more data are needed.
Disclosures
Gavriatopoulou:Genesis Pharma: Honoraria; GSK: Consultancy, Honoraria; Janssen Cilag: Honoraria; Sanofi: Honoraria; Karyopharm: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Terpos:EUSA Pharma: Honoraria, Other: Travel expenses; Amgen: Honoraria, Other: Travel expenses, Research Funding; BMS: Honoraria; Sanofi: Honoraria, Research Funding; Takeda: Honoraria, Other: Travel expenses, Research Funding; Novartis: Honoraria; Janssen: Honoraria, Research Funding; GSK: Honoraria, Research Funding; Genesis: Honoraria, Research Funding. Dimopoulos:BeiGene: Honoraria; Amgen: Honoraria; BMS: Honoraria; Jannsen: Honoraria; TAKEDA: Honoraria. Kastritis:Pfizer: Honoraria, Research Funding; GSK: Honoraria; Genesis Pharma: Honoraria; Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal